Definition, scope, and who this guide is for
The integration of electronics into the human body requires a fundamental shift from standard PCB manufacturing to ultra-high-reliability fabrication. Micro interconnects and flex in implants refer to the specialized category of flexible and rigid-flex circuits designed with high-density interconnects (HDI), extremely tight tolerances, and biocompatible materials intended for operation within a biological environment. These are not merely smaller versions of standard boards; they are engineered systems where failure can result in invasive revision surgery or patient harm.
This playbook is designed for medical device engineers, NPI (New Product Introduction) leads, and procurement managers who are tasked with sourcing these critical components. The scope covers the transition from a prototype design to a scalable, validated manufacturing process. It addresses the unique challenges of miniaturization—where trace widths drop below 3 mils—and the mechanical demands of dynamic flexing inside the body.
We focus on the practicalities of execution: defining the right specifications to prevent drift, identifying manufacturing risks before they become yield losses, and validating that the supplier can meet the stringent cleanliness and reliability standards required for Class II and Class III medical devices. Whether you are developing a neurostimulator, a cochlear implant, or a smart orthopedic sensor, this guide provides the framework to make safe, data-driven sourcing decisions.
Throughout this guide, we will reference the capabilities required to execute these designs, drawing on the manufacturing standards upheld by APTPCB (APTPCB PCB Factory). The goal is to equip you with a checklist and a validation strategy that ensures your micro interconnects and flex in implants perform exactly as simulated, without manufacturing surprises.
When to use this approach (and when not to)
Understanding the specific application environment is the first step in determining if you truly need the complexity of implant-grade micro interconnects. This technology bridges the gap between mechanical constraints and electrical performance.
Use micro interconnects and flex in implants when:
- 3D Geometry Constraints: The device must conform to the curvature of bones, organs, or small enclosures where a rigid board cannot fit.
- Dynamic Movement: The circuit connects sensors or electrodes that move with the body (e.g., a lead connecting a pulse generator to a heart or nerve), requiring a robust
dynamic flex life cycle design. - High I/O in Small Spaces: You need to route hundreds of signals from a high-pin-count ASIC within a footprint smaller than 10mm², necessitating microvias and stacked structures.
- Weight Reduction: The mass of the implant affects patient comfort or device migration; flex circuits significantly reduce weight compared to rigid alternatives and wire harnesses.
Stick to standard rigid or standard flex approaches when:
- External Wearables: If the device is on the skin rather than under it, standard IPC Class 2 or 3 flex specs are often sufficient and more cost-effective.
- Static Applications with Space: If the device is a static implant (like a pacemaker can) with ample internal volume, a standard rigid-flex or small rigid board with wire bonds might be cheaper and simpler to assemble.
- Low Density: If trace widths are above 5 mil and via sizes are standard (0.2mm+), the specialized "micro" processing costs may not be justified.
Specs to define (materials, stackup, tolerances)
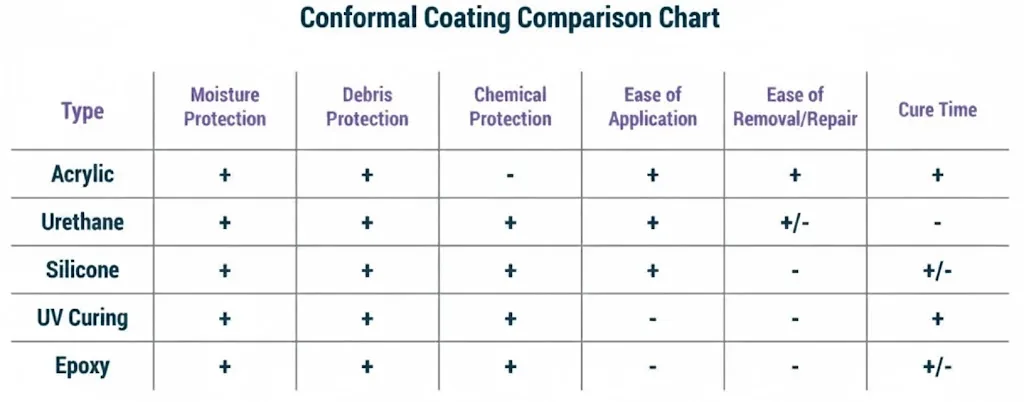
Defining the correct specifications upfront prevents the "engineering query loop" that delays projects. For micro interconnects and flex in implants, the margin for error is non-existent. Below are the baseline specifications you should define in your fabrication drawing.
- Base Material Selection: Specify adhesiveless Polyimide (PI) or Liquid Crystal Polymer (LCP). LCP is increasingly preferred for high-frequency implants due to its low moisture absorption (<0.04%) compared to PI, which is critical for long-term hermeticity.
- Copper Thickness: Utilize ultra-thin copper foils (1/3 oz or 12µm, sometimes down to 5µm) to improve flexibility and allow for finer etch lines. Thicker copper increases stiffness and risk of fatigue cracking.
- Trace and Space: Target 3 mil / 3 mil (75µm) as a standard high-end spec. For true micro interconnects, capabilities may need to push to 2 mil / 2 mil (50µm) or below, requiring Laser Direct Imaging (LDI).
- Microvia Aspect Ratio: Maintain an aspect ratio of 0.8:1 or 1:1 for laser-drilled microvias to ensure reliable plating. Deep, narrow vias are prone to plating voids.
- Surface Finish: Electroless Nickel Immersion Gold (ENIG) or Electroless Nickel Electroless Palladium Immersion Gold (ENEPIG) are standard. Hard gold is required for connector fingers. Ensure the gold thickness is specified to prevent embrittlement or contact failure.
- Coverlay vs. Solder Mask: Use flexible coverlay (PI) rather than flexible solder mask for dynamic regions. Coverlay provides better mechanical protection and dielectric strength.
- Cleanliness Requirements: Specify ionic contamination limits (e.g., <0.50 µg/cm² NaCl equivalent). Implants require stricter cleanliness than standard IPC-6013 Class 3 to prevent dendritic growth and tissue reaction.
- Dimensional Stability: Flex materials shrink and stretch during processing. Specify a tolerance of ±0.05mm for overall outline and ±0.3% for feature-to-feature scaling, and require the manufacturer to apply compensation factors.
- Rigid-Flex Stackup: If using a
rigid-flex PCB stackup design, ensure the transition zone (where rigid meets flex) is reinforced with a bead of epoxy or designed with a "bikini" coverlay to prevent stress concentration. - Impedance Control: If high-speed data is involved, specify differential impedance (usually 100Ω ±10%). Note that cross-hatched ground planes are often used in flex to maintain flexibility while providing shielding.
Manufacturing risks (root causes & prevention)
Manufacturing these components involves aggressive chemical and mechanical processes. Understanding the failure modes helps you audit your supplier effectively.
Microvia Fracture (Barrel Cracks)
- Root Cause: Z-axis expansion mismatch between the dielectric (PI/LCP) and the copper plating during thermal cycling.
- Detection: Interconnect Stress Test (IST) or aggressive thermal shock coupons.
- Prevention: Use materials with matched CTE (Coefficient of Thermal Expansion) and ensure copper plating ductility is high.
Conductor Cracking in Dynamic Flex Areas
- Root Cause: Work hardening of copper due to repeated bending or violation of
flex PCB bend radius rules. - Detection: Flex endurance cycling (IPC-TM-650 2.4.3).
- Prevention: Orient grain direction of rolled annealed (RA) copper along the length of the circuit. Place conductors in the neutral bend axis.
- Root Cause: Work hardening of copper due to repeated bending or violation of
Ionic Contamination (Dendritic Growth)
- Root Cause: Residues from etching, plating chemistries, or handling trapped under coverlay or components.
- Detection: Ion Chromatography (IC) or ROSE testing.
- Prevention: Automated cleaning lines with deionized water and strict cleanroom handling protocols.
Pad Lifting
- Root Cause: Excessive heat during assembly or mechanical stress on small pads without adhesive support.
- Detection: Pull strength test.
- Prevention: Use "anchored" pads (spurs) or larger annular rings where space permits.
Coverlay Misalignment
- Root Cause: Material shrinkage or poor registration during lamination.
- Detection: Visual inspection / AOI.
- Prevention: Laser cutting coverlay openings and using LDI for alignment targets.
Delamination
- Root Cause: Moisture trapped in the polyimide before lamination or poor surface preparation.
- Detection: Thermal stress test (solder float).
- Prevention: Strict baking cycles before lamination and plasma cleaning to activate surfaces.
Plating Voids in Blind Vias
- Root Cause: Trapped air bubbles or insufficient wetting of the via hole during electroless copper deposition.
- Detection: Cross-section analysis.
- Prevention: Ultrasonic agitation and vacuum-assisted plating processes.
Foreign Material Inclusion (FOD)
- Root Cause: Particulates in the lamination press or cleanroom.
- Detection: X-ray or bright light inspection.
- Prevention: Manufacturing in ISO Class 7 or better cleanrooms.
Validation & acceptance (tests and pass criteria)
You cannot rely on standard "pass/fail" electrical testing for implants. You must validate the reliability of the micro interconnects and flex in implants over time.
Objective: Electrical Continuity & Isolation
- Method: Flying probe test at high voltage (250V+ for isolation).
- Criteria: 100% pass. No open/shorts. Isolation resistance >100 MΩ (or as specified).
Objective: Thermal Reliability
- Method: Thermal Shock (-55°C to +125°C, 100+ cycles).
- Criteria: Change in resistance <10%. No delamination or via cracks in microsections.
Objective: Plating Integrity
- Method: Microsectioning (coupon analysis) per IPC-6013 Class 3.
- Criteria: Copper thickness meets spec (e.g., min 20µm in hole). No knee cracks, no separation of internal layers.
Objective: Cleanliness / Biocompatibility Proxy
- Method: Ion Chromatography.
- Criteria: Total ionic contamination <0.50 µg/cm² NaCl equivalent. Specific limits for chloride, bromide, and sulfate.
Objective: Dynamic Flexibility
- Method: MIT Folding Endurance Test.
- Criteria: Withstand X cycles (e.g., 100,000) at Y bend radius without electrical discontinuity.
Objective: Solderability
- Method: Solder float test / Wetting balance.
- Criteria: >95% coverage, no dewetting.
Objective: Dimensional Accuracy
- Method: CMM (Coordinate Measuring Machine) or Optical Vision System.
- Criteria: All critical dimensions within tolerance (typically ±0.05mm).
Objective: Impedance Verification
- Method: TDR (Time Domain Reflectometry) on test coupons.
- Criteria: Measured impedance within ±10% of design target.
Supplier qualification checklist (RFQ, audit, traceability)
When evaluating a partner like APTPCB, use this checklist to ensure they have the specific infrastructure for implantable electronics.
Group 1: RFQ Inputs (What you must provide)
- Gerber files (RS-274X or ODB++) with clear layer stackup.
- Fabrication drawing specifying IPC-6013 Class 3 (or Class 3/A for space/military/implant).
- Material datasheets (or specific callouts for LCP/PI grades).
- Netlist for electrical verification.
- Panelization requirements (if assembly is automated).
- 3D STEP model (crucial for rigid-flex to visualize folding).
- Specific cleanliness and packaging requirements (e.g., vacuum sealed, ESD safe).
Group 2: Capability Proof (What they must demonstrate)
- Demonstrated ability to plate microvias (laser drilled) with aspect ratios >0.8:1.
- LDI (Laser Direct Imaging) capability for <3 mil trace/space.
- Plasma cleaning equipment (essential for desmear and activation).
- Vacuum lamination presses (to prevent voids in rigid-flex).
- Laser cutting/routing for precise flex outlines.
- In-house microsection lab for immediate feedback.
Group 3: Quality System & Traceability
- ISO 13485 certification (Medical Devices Quality Management).
- Lot traceability down to the raw material roll/sheet.
- Chemical process control records (bath analysis logs).
- Calibration records for all measurement and test equipment.
- CAPA (Corrective and Preventive Action) system evidence.
- Record retention policy (typically 5-10 years for medical).
Group 4: Change Control & Delivery
- Strict PCN (Process Change Notification) policy—no changes without approval.
- "Copy Exact" philosophy for recurring builds.
- Secure data handling (IP protection).
- Disaster recovery plan for manufacturing continuity.
Decision guidance (trade-offs and decision rules)
Engineering is the art of compromise. Here is how to navigate the trade-offs in micro interconnects and flex in implants.
Flexibility vs. Layer Count:
- Rule: If you need extreme dynamic flexibility (millions of cycles), keep the flex section to 1 or 2 layers maximum.
- Trade-off: If you need more routing layers, you must accept a larger bend radius or move to a static flex design.
Cost vs. Miniaturization:
- Rule: If you prioritize cost, stick to 3 mil trace/space and mechanical drilling (0.15mm vias).
- Trade-off: If you prioritize miniaturization (2 mil trace, 0.075mm laser vias), cost will increase by 30-50% due to yield impact and laser time.
LCP vs. Polyimide:
- Rule: If you prioritize high-frequency performance (>10GHz) or near-hermetic moisture resistance, choose LCP.
- Trade-off: LCP is harder to process (lamination temperature sensitivity) and more expensive than standard Polyimide.
Stiffeners vs. Integration:
- Rule: If you prioritize connector reliability, use FR4 or Polyimide stiffeners at connection points.
- Trade-off: Stiffeners add manual assembly steps and thickness.
Surface Finish Reliability:
- Rule: If you prioritize wire bonding, use ENEPIG or Soft Gold.
- Trade-off: Standard ENIG is cheaper but risks "black pad" if not controlled perfectly, which is unacceptable for implants.
FAQ (cost, lead time, Design for Manufacturability (DFM) files, testing)
Q: What are the primary cost drivers for micro interconnects and flex in implants?
- Answer: The main drivers are layer count (especially in rigid-flex), the use of laser-drilled microvias, and specialized materials like LCP.
- Details:
- Rigid-flex is typically 3-5x the cost of rigid PCBs.
- Strict Class 3 inspection and cross-sectioning add NRE (Non-Recurring Engineering) costs.
- Yield loss on ultra-fine traces (<3 mil) impacts unit price.
Q: How does lead time for implant-grade flex differ from standard PCBs?
- Answer: Lead times are longer, typically 15-25 working days for prototypes, due to the complexity of lamination cycles and extensive testing.
- Details:
- Standard rigid: 3-5 days.
- Implant flex: Requires plasma treatment, multiple lamination presses, and cure cycles.
- Material availability (e.g., specific LCP thickness) can add weeks if not stocked.
Q: What specific DFM files are needed for micro interconnects and flex in implants?
- Answer: Beyond Gerbers, you must provide a detailed stackup drawing showing material types, grain direction, and stiffener locations.
- Details:
- ODB++ is preferred as it contains intelligent data.
- Define "bikini" coverlay zones clearly.
- Include a netlist to verify data integrity before CAM engineering.
Q: Can standard FR4 be used in any part of micro interconnects and flex in implants?
- Answer: FR4 is often used as a stiffener or in the rigid sections of a rigid-flex design, but it must be sealed or encapsulated if exposed to body fluids.
- Details:
- FR4 is hygroscopic and not biocompatible on its own.
- For the flex section, only PI or LCP should be used.
Q: What are the acceptance criteria for micro interconnects and flex in implants testing?
- Answer: Acceptance is based on IPC-6013 Class 3, but often augmented with customer-specific reliability tests like IST and ionic cleanliness limits.
- Details:
- Zero open/shorts allowed.
- Visual inspection at 10x-40x magnification.
- Pass/Fail on thermal shock coupons is mandatory for lot release.
Q: How do flex PCB bend radius rules apply to implantable devices?
- Answer: The standard IPC rule (10x thickness for dynamic, 20x for static) is a minimum; implants often require more conservative ratios to ensure longevity.
- Details:
- For dynamic applications, aim for 20x-40x thickness to bend radius ratio.
- Use rolled annealed copper for dynamic flexing.
Q: Why is rigid-flex PCB stackup design critical for implants?
- Answer: An unbalanced stackup causes warping and delamination, which compromises the hermetic seal or mechanical fit of the implant.
- Details:
- Symmetrical construction reduces stress.
- Adhesiveless base materials prevent outgassing and improve reliability.
Q: What materials are best for dynamic flex life cycle design in implants?
- Answer: Adhesiveless Polyimide with Rolled Annealed (RA) copper is the gold standard for high-cycle dynamic flexing.
- Details:
- Avoid electrodeposited (ED) copper in dynamic zones (it fractures easily).
- Coverlays should be used instead of solder mask to prevent cracking.
Related pages & tools
- Rigid-Flex PCB Capabilities – Explore the manufacturing limits and stackup options for combining rigid stability with flexible routing.
- Medical PCB Industry Solutions – Understand the specific quality standards and certifications APTPCB applies to medical device manufacturing.
- Flex PCB Technology – A deep dive into flexible circuit materials, bend radius calculations, and dynamic flex applications.
- HDI PCB Manufacturing – Learn about microvias, fine lines, and high-density interconnects essential for miniaturized implants.
- PCB Quality System – Review the testing protocols, including microsectioning and electrical testing, that ensure zero-defect delivery.
- DFM Guidelines – Access design rules to optimize your implantable PCB for manufacturability and yield.
Request a quote (Design for Manufacturability (DFM) review + pricing)
Getting an accurate quote for implantable electronics requires more than just a file upload; it requires a technical review to ensure the stackup and materials are viable for mass production. At APTPCB, our engineering team reviews your data against our Class 3 medical manufacturing capabilities to identify risks before pricing.
To expedite your quote, please provide:
- Gerber Files / ODB++: Complete data package.
- Fabrication Drawing: Including material specs (PI/LCP), stackup, and tolerances.
- Volume & EAU: Prototype quantity vs. production targets.
- Testing Requirements: Specific cleanliness levels or custom testing protocols.
Click here to Request a Quote & DFM Review – We typically respond with a preliminary DFM report and pricing within 24-48 hours.
Conclusion (next steps)
Successfully deploying micro interconnects and flex in implants is a rigorous process that demands precise engineering and a transparent supplier partnership. By defining robust specifications for materials and stackups, understanding the root causes of manufacturing risks like microvia fractures, and enforcing a strict validation checklist, you can secure the reliability of your medical device. Whether you are in the prototyping phase or scaling for clinical trials, the focus must remain on repeatable quality and traceability. Use the guidelines and checklists provided here to align your design with manufacturing realities, ensuring your implantable technology performs safely for the patient.