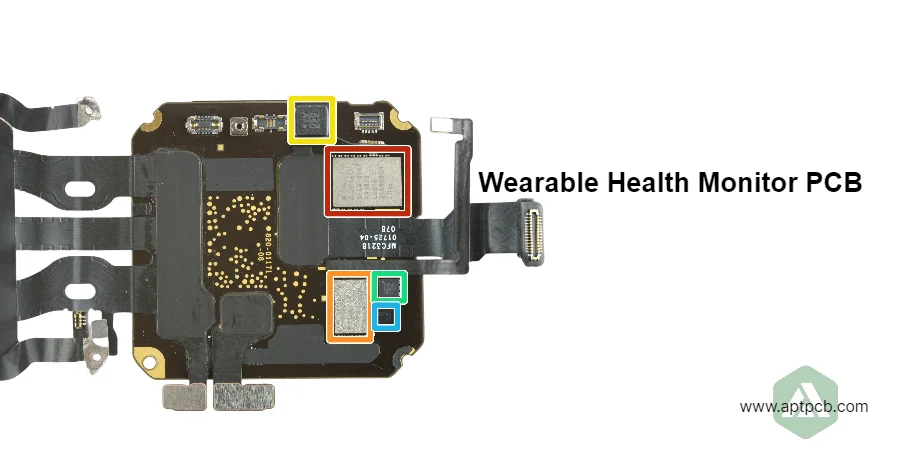
Wearable health monitor PCB assemblies integrate biosensors (PPG, ECG, accelerometer), ultra-low-power processors, wireless connectivity, and battery management supporting continuous health tracking, patient monitoring, and medical-grade diagnostics requiring <20mAh daily power consumption, clinical measurement accuracy, biocompatible construction, and regulatory compliance across consumer fitness bands, medical wearables, and remote patient monitoring platforms operating continuously for days to weeks between charges through 2-5 year product lifecycles.
At APTPCB, we deliver wearable health assembly services with biosensor expertise, ultra-low-power design, and medical validation supporting box build assembly from consumer wellness through FDA-cleared medical devices.
Implementing Clinical-Grade Biosensors
Medical wearables measure heart rate, SpO2, ECG, and activity requiring precision optical sensors (PPG), biopotential electrodes, and motion sensors achieving clinical accuracy despite challenging measurement conditions on moving users. Biosensor challenges include motion artifact rejection, ambient light interference, and skin tone variations affecting optical measurements. Inadequate sensor implementation causes inaccurate metrics, false alarms, or regulatory compliance failures — significantly impacting clinical utility and FDA clearance.
At APTPCB, our assembly integrates precision biosensors achieving medical-grade measurement accuracy.
Biosensor Integration
- PPG Optical Sensors: Multi-wavelength LEDs and photodiodes with ambient light rejection achieving ±2bpm heart rate and ±2% SpO2 accuracy with ICT test validation.
- ECG Biopotential Amplifiers: High input impedance (>10MΩ) instrumentation amplifiers with right-leg drive enabling single-lead ECG measurement.
- Motion Sensor Fusion: 6-axis IMU combining accelerometer and gyroscope detecting activity, sleep, and fall events with validated algorithms.
- Clinical Validation: Correlation studies against gold-standard medical equipment demonstrating accuracy across user populations and skin tones.
Achieving Ultra-Low-Power Operation
Battery-powered wearables require <20mAh daily consumption from 100-300mAh batteries achieving multi-day runtime through aggressive duty cycling, efficient wireless protocols, and optimized firmware. Power challenges include maintaining wireless connectivity during sleep, balancing sensor sampling rates against battery life, and achieving regulatory requirements with limited battery capacity. Inadequate power management causes frequent charging frustrating users, limits continuous monitoring capability, or prevents overnight operation — significantly impacting user adoption and clinical utility for continuous health tracking.
At APTPCB, our manufacturing supports ultra-low-power designs achieving extended battery runtime.
Power Optimization
- Ultra-Low-Power MCUs: ARM Cortex-M0+ consuming <10μA sleep while maintaining RTC and wake capabilities through NPI assembly characterization.
- Duty-Cycled Sensing: Periodic PPG measurements (every 5-10s) and motion-triggered ECG recording minimizing active time while maintaining clinical utility.
- Efficient Wireless: BLE 5.0 connection intervals optimized for latency versus power balancing responsiveness against battery consumption.
- Dynamic Power Management: Adaptive operation adjusting sampling rates and features based on remaining battery capacity extending operation during low battery.
Ensuring Biocompatibility and Skin Safety
Continuous skin contact requires biocompatible materials, hypoallergenic coatings, and skin irritation testing preventing adverse reactions during 24/7 wear. Biocompatibility challenges include selecting body-safe materials, preventing nickel exposure, and validating long-term skin contact safety. Inadequate biocompatibility causes skin rashes limiting user adoption, allergic reactions reducing addressable market, or regulatory failures preventing clearance — significantly impacting commercial success and patient safety.
At APTPCB, our manufacturing implements biocompatible processes supporting continuous-wear medical wearables.
Biocompatibility Implementation
- Medical-Grade Materials: Biocompatible PCB laminates and conformal coatings meeting ISO 10993 cytotoxicity and sensitization requirements.
- Nickel Barrier: ENIG surface finish with adequate gold thickness preventing nickel exposure to sensitive users.
- Hypoallergenic Coatings: Medical-grade parylene or urethane encapsulation enabling safe continuous skin contact.
- ISO 10993 Testing: Comprehensive biocompatibility testing validating safety for intended 24/7 wear duration.
Through biocompatible materials and validated testing coordinated with mass production quality control, APTPCB enables wearables safe for continuous patient contact.
Supporting FDA Medical Device Compliance
Medical wearables providing diagnostic information or disease monitoring require FDA 510(k) clearance or De Novo classification demonstrating safety, effectiveness, and clinical validation. FDA challenges include meeting electrical safety standards, demonstrating clinical accuracy, and maintaining design controls. Inadequate compliance prevents medical claims, limits reimbursement eligibility, or restricts clinical adoption — significantly affecting market opportunity and revenue potential.
At APTPCB, we support FDA-cleared medical wearable development with regulatory expertise.
FDA Compliance Support
- Design Controls: ISO 13485 processes covering requirements, verification, validation, and risk management per 21 CFR 820.
- Clinical Studies: Correlation studies and clinical trials demonstrating measurement accuracy and clinical utility for intended use.
- Electrical Safety: IEC 60601-1 compliance for patient-connected wearables ensuring protection against electrical hazards.
- Regulatory Submissions: 510(k) preparation including predicate identification, substantial equivalence demonstration, and submission support.
Through FDA regulatory expertise and quality system compliance, APTPCB enables medical wearable manufacturers achieving clearance and market access.
Integrating Wireless Connectivity and Data Security
Medical wearables transmit protected health information requiring encrypted wireless communication, secure data storage, and HIPAA compliance protecting patient privacy. Security challenges include implementing secure pairing, preventing eavesdropping, and achieving compliance with limited processor resources. Inadequate security exposes patient data, enables unauthorized access, or fails compliance audits — significantly impacting trust, regulatory standing, and commercial partnerships with healthcare providers.
At APTPCB, our assembly supports secure medical wearables protecting patient data and privacy.
Security Implementation
- Encrypted Communication: BLE secure connections with AES-128 encryption protecting data transmission from wearable to smartphone.
- Secure Storage: Encrypted flash memory protecting health data stored on device preventing unauthorized access.
- Secure Boot: Cryptographic boot verification preventing unauthorized firmware modification ensuring device integrity.
- HIPAA Compliance: Technical safeguards meeting HIPAA Security Rule requirements for protected health information.
Through security implementation and compliance expertise coordinated with component sourcing of qualified components, APTPCB enables medical wearables meeting healthcare privacy and security requirements.